Since 2016, when the United States Food and Drug Administration approved the iLink® cross-linking technology, more patients are asking us, “What is cross-linking?” It sounds like a type of fence or a sewing technique – but for patients at Eye Specialty Group, it is so much more significant than that.
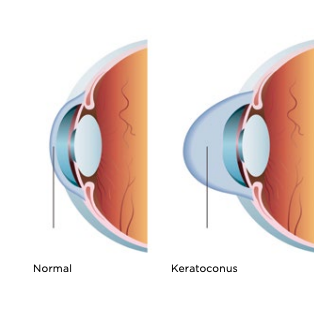
Cross-linking is an outpatient, minimally invasive surgery that is used to treat a weakened or warped cornea, a condition called keratoconus. Disease, age, inflammation and injury can all contribute to the breakdown of collagen in the cornea, which plays a vital role in holding it together. This weakening of tissue allows the cornea to bulge out, which causes blurriness and other visual disturbances. “Cross-linking” is designed to stop the progression of keratoconus by strengthening the bonds between collagen fibers of the eye, thus stabilizing the cornea.
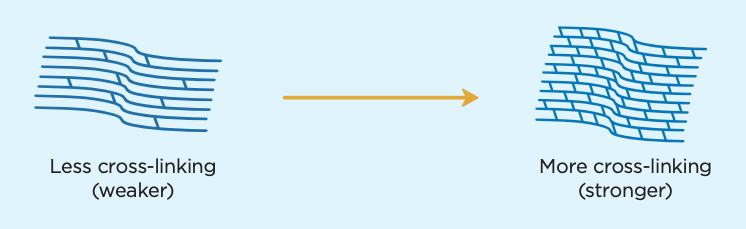
Now that you know what cross-linking is, let’s review what it treats and how it works.
Cross-linking is commonly used to treat keratoconus (KC). This condition is when the cornea thins and weakens, causing a bulge that distorts vision. Cross-linking slows the progression of KC by creating new collagen connections to reinforce the cornea. Think of these new bonds as support beams for the eye.
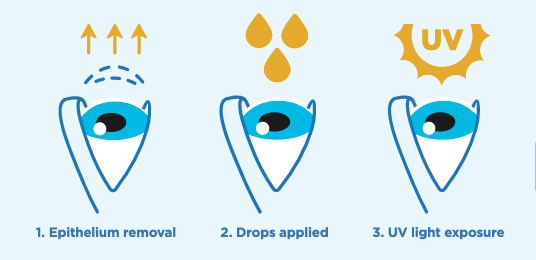
Cross-linking uses ultraviolet light and specially formulated eye drops together to increase the number of molecular bonds, or cross-links, in the collagen of the cornea. This stiffens the cornea and slows – or even stops – the progression of keratoconus. The cross-linking procedure is quick and straightforward:
During the first week of healing, it’s important to take it easy in a clean environment and NOT rub your eyes, get water in your eyes or use makeup near your eyes.
There is no pain during cross-linking, thanks to numbing drops that are applied before treatment starts. Once the anesthetic drops wear off, patients may experience mild burning or a gritty sensation, which can usually be managed with acetaminophen and artificial tears.
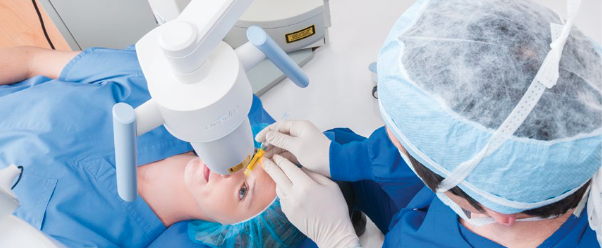
iLink® is the first corneal cross-linking treatment to receive FDA approval. This is significant because insurance doesn’t typically cover products and procedures that lack FDA approval or that have not been proven safe and effective in clinical trials. Thus, iLink is the first cross-linking procedure covered by insurance.
Eye doctors and patients love iLink corneal cross-linking for a variety of reasons, including clinically proven safety and effectiveness in protecting the eye from progressive keratoconus damage.
If you suffer from keratoconus, talk to a doctor with Eye Specialty Group about iLink® cross-linking treatment to preserve your vision.
Call (901) 685-2200 or tap here to request an appointment.
1000 Windover Rd. Suite C
Jonesboro, AR 72401
Hours:
M-F: 7:30am - 5pm
1458 W Poplar, Suite 101
Collierville, TN 38017
Hours:
M-F: 7:30am - 5pm
7600 Airways Blvd. Suite F
Southaven, MS 38671
Hours:
M-F: 7:30am - 5pm